Always consider drug toxicity in the presence of jaundice or abnormal liver biochemistry. This often means obtaining a detailed drug history from the GP and asking family members about herbal remedies and over-the-counter medication. Acute liver failure has frequently been reported following anti-tuberculous therapy. Although there is usually a very clear temporal association between taking the drug and the onset of hepatotoxicity, halothane and co-amoxiclav may present up to three weeks after taking the drug. Prognosis is worse than with liver failure secondary to acute viral hepatitis.
While the mainstay of treatment is supportive, the need for transplanataion should be borne in mind, and early discussions should take place with a transplant unit.
Showing posts with label hepatology. Show all posts
Showing posts with label hepatology. Show all posts
Chronic Hepatitis B
Government estimates are that about 180,000 people in the UK have chronic hepatitis B and the prevalence of hepatitis B surface antigen (HBsAg) among blood donors is around 1 in 1,500. Around one-fifth of HBsAg-positive individuals are HBeAg positive, with 7-20% spontaneously losing HBeAg positivity per year.
There are five therapies for chronic hepatitis B that are currently approved:
Students found to be infected with a blood-borne virus such as hepatitis B are allowed to continue their medical course leading to full medical registration provided they accept the requirement that they will not be allowed to perform exposure-prone procedures while infectious (HBeAg positive or HBeAg negative with >103 genome equivalents/mL HBV DNA), and that careers in some specialties may not be open to them. Registered medical students who are HBeAg negative and who have virus loads that do not exceed 103 genome equivalents/mL will be tested annually in order to confirm that their virus load has not exceeded the threshold at which they would be prohibited from conducting exposure-prone procedures. Those who have undergone a course of treatment need to show that they have a viral load that does not exceed 103 genome equivalents/mL 1 year after cessation of treatment before a return to unrestricted working practices can be considered.
There are five therapies for chronic hepatitis B that are currently approved:
- interferon alfa-2b,
- lamivudine,
- adefovir,
- entecavir and
- pegylated interferon (peginterferon) alfa-2a.
Students found to be infected with a blood-borne virus such as hepatitis B are allowed to continue their medical course leading to full medical registration provided they accept the requirement that they will not be allowed to perform exposure-prone procedures while infectious (HBeAg positive or HBeAg negative with >103 genome equivalents/mL HBV DNA), and that careers in some specialties may not be open to them. Registered medical students who are HBeAg negative and who have virus loads that do not exceed 103 genome equivalents/mL will be tested annually in order to confirm that their virus load has not exceeded the threshold at which they would be prohibited from conducting exposure-prone procedures. Those who have undergone a course of treatment need to show that they have a viral load that does not exceed 103 genome equivalents/mL 1 year after cessation of treatment before a return to unrestricted working practices can be considered.
Management of Hepatitis
Ribavirin has no effect on hepatitis B replication, but used in combination with interferon is more effective than ribavirin alone in eradicating chronic infection with hepatitis C.
Interferon was until recently first line treatment for chronic hepatitis B infection associated with elevated serum transaminases. However, there is often a flare in ALT (alamine aminotransferase) on starting treatment, which in the presence of end stage liver disease (elevated PT, bilirubin and ascites here) can lead to liver failure so, in these circumstances, it is contra-indicated.
Lamivudine suppresses HBV replication and is safe to use in decompensated end-stage cirrhosis.
Reference
Interferon was until recently first line treatment for chronic hepatitis B infection associated with elevated serum transaminases. However, there is often a flare in ALT (alamine aminotransferase) on starting treatment, which in the presence of end stage liver disease (elevated PT, bilirubin and ascites here) can lead to liver failure so, in these circumstances, it is contra-indicated.
Lamivudine suppresses HBV replication and is safe to use in decompensated end-stage cirrhosis.
Reference
- Yao FY, Terrault NA, Freise C, Maslow L, Bass NM. Lamivudine treatment is beneficial in patients with severly decompensated cirrhosis and actively replicating hepatitis B viral infection awaiting liver transplantation: a comparative study using matched untreated controls. Hepatology 2001; 34: 411-6
Risk factors for hepatocellular carcinoma (HCC)
Eighty per cent of cases of hepatocellular carcinoma (HCC) are associated with chronic hepatitis B infection. Most cases of HCC not associated with hepatitis B infection are associated with hepatitis C infection.
Alcoholic cirrhosis and autoimmune chronic active hepatitis also increase the risk of developing HCC.
Several metabolic diseases are also associated with an increased risk for the development of HCC, such as haemochromatosis (iron accumulation), Wilson's disease (copper accumulation), alpha-1-antitrypsin deficiency and glycogen storage diseases.
Alflatoxins are mycotoxins produced by the fungi Aspergillus flavus and Aspergillus parasiticus which contaminate food (e.g. peanuts and corn).
A less common association is with primary biliary cirrhosis which is more typically associated with the subsequent development of cholangiocarcinoma.
Reference
Alcoholic cirrhosis and autoimmune chronic active hepatitis also increase the risk of developing HCC.
Several metabolic diseases are also associated with an increased risk for the development of HCC, such as haemochromatosis (iron accumulation), Wilson's disease (copper accumulation), alpha-1-antitrypsin deficiency and glycogen storage diseases.
Alflatoxins are mycotoxins produced by the fungi Aspergillus flavus and Aspergillus parasiticus which contaminate food (e.g. peanuts and corn).
A less common association is with primary biliary cirrhosis which is more typically associated with the subsequent development of cholangiocarcinoma.
Reference
- DeVita VT, Hellman S, Rosenberg SA. Cancer Principles and Practice of Oncology, 5th edn. Philadelphia: Lippincott-Raven.
Portal vein
About 75% of the blood supply to the liver comes from the portal vein, which is formed by the union of superior mesenteric and splenic veins.
Inside the liver, blood from the portal vein and from the hepatic artery flows through the tortuous capillaries called sinusoids. The venous outflow to the inferior vena cava from the liver is via the hepatic veins and obstruction to this outflow causes Budd–Chiari syndrome.
The normal portal pressure is about 5-8mm Hg: in portal hypertension it rises above 10–12mm Hg.
Inside the liver, blood from the portal vein and from the hepatic artery flows through the tortuous capillaries called sinusoids. The venous outflow to the inferior vena cava from the liver is via the hepatic veins and obstruction to this outflow causes Budd–Chiari syndrome.
The normal portal pressure is about 5-8mm Hg: in portal hypertension it rises above 10–12mm Hg.
Interferon-alfa and Ribavirin for Chronic Hepatitis C
Combination therapy with interferon-alfa and ribavirin is generally recommended for those with moderate-sever disease (histological diagnosis of significant scarring and/or significant necrotic inflammation). While NICE guidance suggests that problems with drug interactions, safety, and compliance may arise in existing intravenous drug users, those who have given up the habit should not be excluded from therapy. However, treatment is not generally recommended in those patients who consume large quantities of alcohol, given the increased risk of liver damage.
In cases where a liver biopsy carries a high risk (e.g. haemophilia), treatment can be initiated without histological confirmation.
Both treatment-naïve patients and those who have relapsed following initial response to interferon-alfa should be considered for 6 months of combination therapy.
In cases where a liver biopsy carries a high risk (e.g. haemophilia), treatment can be initiated without histological confirmation.
Both treatment-naïve patients and those who have relapsed following initial response to interferon-alfa should be considered for 6 months of combination therapy.
Drug-induced Liver injury
Virtually all drugs can cause liver injury. In this case the most recently introduced medication is most likely to be the cause of liver injury, assuming other investigations for chronic liver disease are negative. The prescription drugs to be particularly aware of when assessing a patient with an undiagnosed liver disorder include Augmentin (co-amoxiclav), diclofenac, isoniazid, erythromycin, sodium valproate, amiodarone and phenytoin.
The Biliary Tract
The right and left hepatic ducts join together to form the common hepatic duct, which in turn is joined by the cystic duct to form the bile duct in the free border of the lesser omentum. The bile duct then passes behind the first part of the duodenum and the head of the pancreas, joins with the pancreatic duct to form the ampulla of Vater, and opens into the second part of the duodenum on its posteromedial wall on the papilla of Vater.
The papilla is 10 cms distal to the pylorus.
The sphincter of Oddi surrounds the ampulla as well as the ends of the common bile duct and pancreatic ducts.
The papilla is 10 cms distal to the pylorus.
The sphincter of Oddi surrounds the ampulla as well as the ends of the common bile duct and pancreatic ducts.
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of diseases that includes simple steatosis, steatohepatitis, advanced fibrosis and cirrhosis. The diagnosis should be suspected in patients with persistently elevated alanine aminotransferase (ALT) values with:
▪ negative screening for viral hepatitis
▪ autoimmune hepatitis and metabolic liver disease
▪ no high risk alcohol or medication use
▪ fatty infiltration on ultrasound.
NAFLD is associated with the metabolic syndrome (obesity, insulin resistance, hyperlipidaemia and hypertension) and is therefore often seen in obese patients, who may have impaired glucose intolerance.
Treatment and prognosis is unclear, but attention to modifiable risk factors is important including weight loss, diabetic management and treatment of hyperlipidaemia.
▪ negative screening for viral hepatitis
▪ autoimmune hepatitis and metabolic liver disease
▪ no high risk alcohol or medication use
▪ fatty infiltration on ultrasound.
NAFLD is associated with the metabolic syndrome (obesity, insulin resistance, hyperlipidaemia and hypertension) and is therefore often seen in obese patients, who may have impaired glucose intolerance.
Treatment and prognosis is unclear, but attention to modifiable risk factors is important including weight loss, diabetic management and treatment of hyperlipidaemia.
Cytomegalovirus (CMV) Infection
Cytomegalovirus (CMV) infection is an important consideration in any organ transplant recipient, particularly where the recipient has never had infection but the donor is a carrier, since the recipient is then at risk of primary infection. For this reason close attention is given to prophylaxis, particularly in the first 3 months after transplantation. When prophylaxis is stopped there is still a chance of infection, which in the case of liver transplant recipients will often present as fever, abdominal pain, diarrhoea (colitis), breathlessness (pneumonitis) and hepatitis. Other features of the illness can include haematological abnormalities, retinitis and oesophagi tis.
Diagnosis is by quantifying CMV viraemia in the blood. Treatment is with intravenous ganciclovir and reduction of immunosuppression if possible.
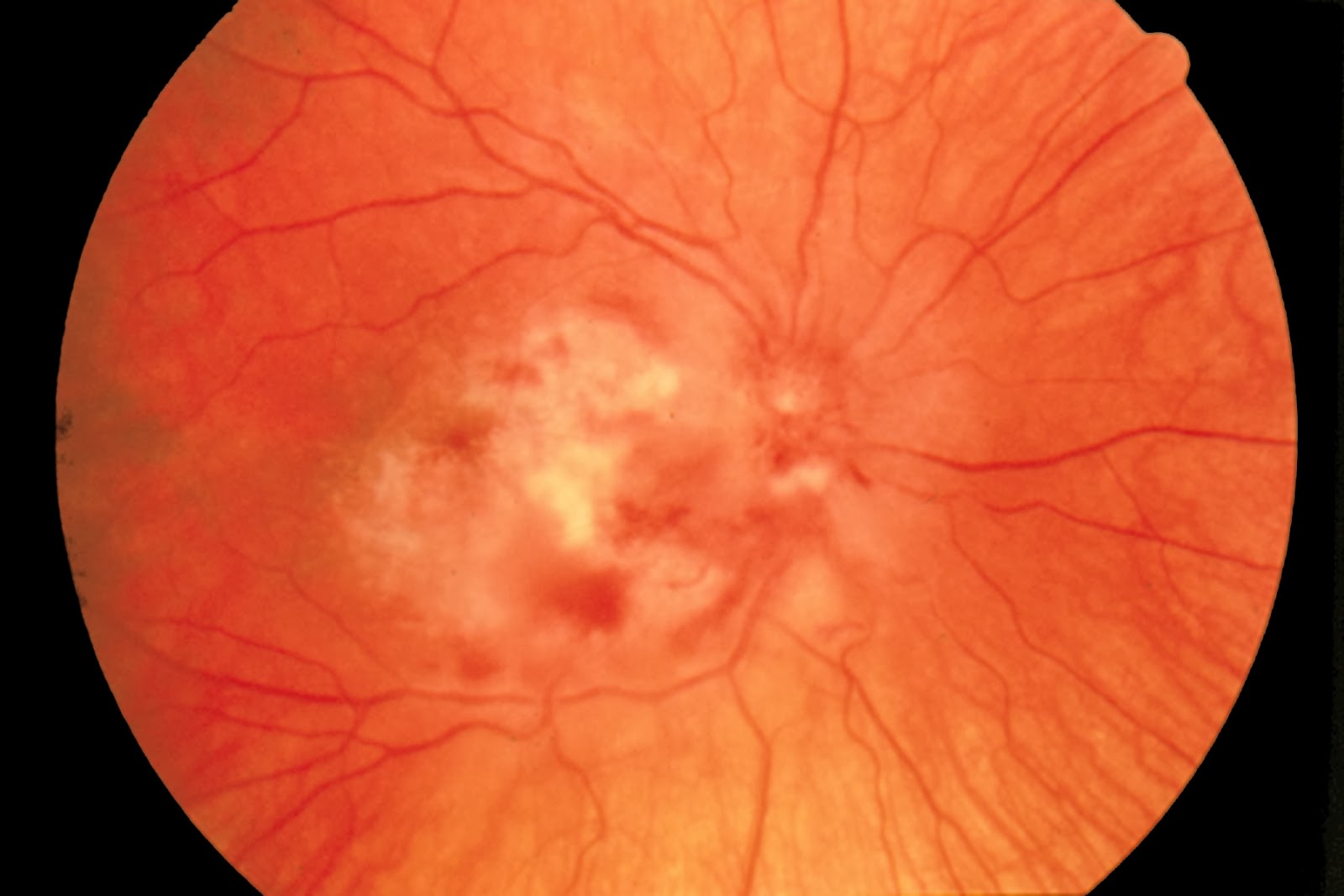 |
| Fig 2: CMV retinitis. |
Subscribe to:
Posts (Atom)
